The first pill to treat adults with severe alopecia was approved by the U.S. Food and Drug Administration on Monday.
Olumiant (baricitinib) is the first FDA-approved alopecia therapy that treats the entire body rather than a specific spot, the agency said in a news release announcing the approval.
“Access to safe and effective treatment options is crucial for the significant number of Americans affected by severe alopecia,” Dr. Kendall Marcus, director of the Division of Dermatology and Dentistry in the FDA’s Center for Drug Evaluation and Research at the FDA, said in the news release. “Today’s approval will help fulfill a significant unmet need for patients with severe alopecia areata.”
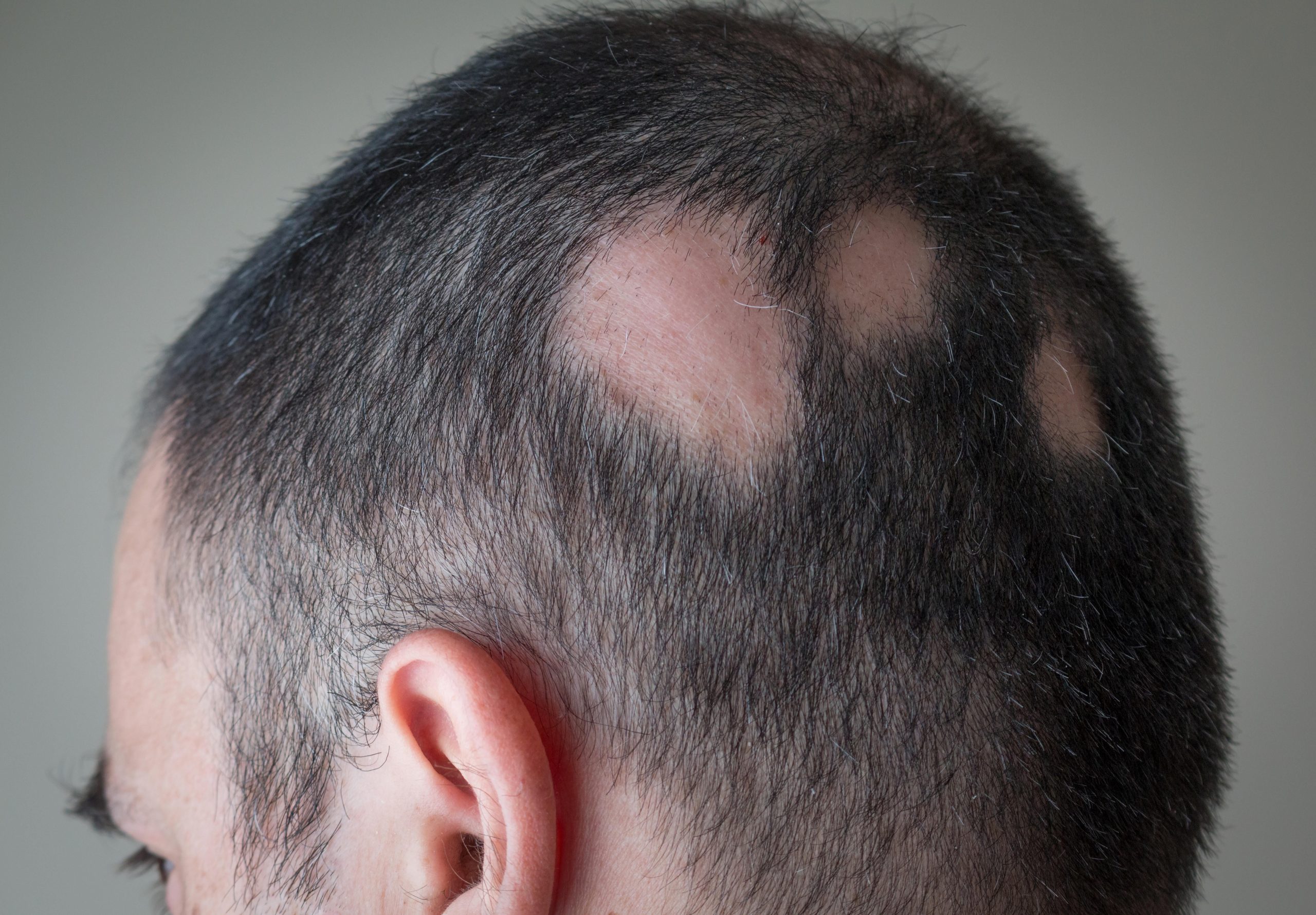
Alopecia areata is an autoimmune disorder in which the body attacks its own hair follicles, causing hair to fall out, often in clumps. It affects more than 300,000 people in the United States each year, according to the FDA.
One of those is actress Jada Pinkett Smith, who first revealed her struggles with hair loss in 2018.
For most people, the disease involves one or a few small bald patches on the head. But those with severe cases may notice small bald spots on their heads one day, and then they no longer have any hair on their bodies three months, or even three weeks, later.
Take the case of Christian Daniels. The 27-year-old data center technician from Peoria, Ill., said his hair started falling out when he was 25. Within a month, all of his body hair was gone.
Even his vision was affected: Without eyelashes, dust would get into his eyes and irritate them so much he began putting Vaseline on his eyelids.
The pandemic was a “blessing in disguise” because he could work at home.
“I felt like my life had been put on hold,” he told The New York Times. “I felt like the only thing that mattered was how to get my hair back.”
Now, after being part of a trial of the drug that prompted the FDA approval, “It’s almost like it [alopecia] never happened,” he said, although he still looks in a mirror sometimes and has a flashback to his hairless self.
Olumiant is a Janus kinase (JAK) inhibitor that works by interfering with the cellular pathway that triggers inflammation. It was first approved in 2018 to treat rheumatoid arthritis, the FDA said.
The agency’s approval of the drug from Eli Lilly and Co. is based on two clinical trials that included 1,200 alopecia patients with at least 50% hair loss who took either 2 or 4 milligrams of Olumiant or a placebo every day.
After 36 weeks, rates of patients who achieved at least 80% hair coverage were 17% and 22% for those who took 2 milligrams of Olumiant and 32% and 35% of those who took 4 milligrams of the drug. That compared with 3% and 5% of those who took a placebo, according to the FDA.
The most common side effects associated with Olumiant included: upper respiratory tract infections, headache, acne, high cholesterol, increase of an enzyme called creatinine phosphokinase, urinary tract infection, liver enzyme elevations, inflammation of hair follicles, fatigue, lower respiratory tract infections, nausea, genital yeast infections, anemia, low number of certain types of white blood cells, abdominal pain, shingles and weight gain.
Olumiant should not be used in combination with other JAK inhibitors or any other potent immunosuppressants, the FDA warned. The drug carries a boxed warning for serious infections, death, cancer, major heart problems and blood clots.
Patient taking the drug should be closely monitored for infection during and after treatment and should be checked for latent and active tuberculosis before treatment, the FDA advised.
With the FDA approval will come insurance coverage for these expensive drugs, which have a list price of nearly $2,500 a month, The New York Times reported. Two other companies, Pfizer and Concert Pharmaceuticals, are close behind Lilly with similar drugs that are already on the market for the treatment of rheumatoid arthritis and other autoimmune diseases.
More information
There’s more on alopecia at the U.S. National Library of Medicine.
SOURCES: U.S. Food and Drug Administration, news release, June 13, 2022
Source: HealthDay
Copyright © 2024 HealthDay. All rights reserved.

Leave a Reply