
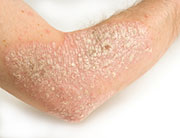
WEDNESDAY, Sept. 30, 2015Two experimental drugs show promise in treating psoriasis and a related condition, psoriatic arthritis, new studies report.
The drugs, brodalumab and secukinumab (Cosentyx), represent a new approach to treatment, said Michael Siegel, director of research programs at the National Psoriasis Foundation.
“These studies show how targeting parts of the immune system can have great effects, and that’s really exciting for our patients,” said Siegel, who wasn’t involved in the research.
Psoriasis, a chronic autoimmune condition, causes raised red patches of skin topped with silvery scales. These patches usually appear on the scalp, elbows, knees, face, lower back, hands and feet. Psoriatic arthritis is a form of the disease that includes joint pain, stiffness and swelling.
The study findings appear in the Oct. 1 issue of the New England Journal of Medicine.
In one study, brodalumab reduced psoriasis symptoms 100 percent in more than 40 percent of patients. In the other report, Cosentyx slowed progression of psoriatic arthritis.
“Brodalumab was dramatically able to clear psoriasis,” said the lead researcher of that study, Dr. Mark Lebwohl, chairman of dermatology at the Icahn School of Medicine at Mount Sinai in New York City.
Brodalumab is a so-called monoclonal antibody designed to block the function of a protein called interleukin 17 (IL-17), which contributes to psoriasis, Lebwohl explained.
For the phase 3 brodalumab trials — the final phase needed for U.S. drug approval — researchers randomly selected more than 3,000 patients with moderate to severe psoriasis to receive either brodalumab, ustekinumab (Stelara) or placebo. According to Lebwohl, Stelara is currently the best psoriasis drug available.
Forty-four percent of patients using brodalumab had 100 percent of their psoriasis cleared, compared with 22 percent of those receiving Stelara, Lebwohl said.
Moreover, more than 68 percent of patients receiving brodalumab saw 90 percent of their psoriasis clear, compared with 47 percent of patients receiving Stelara, he added.
The study was funded by drug maker Amgen, which co-developed brodalumab with AstraZeneca.
Brodalumab is injected every two weeks. Because psoriasis is a chronic disease, treatment lasts a lifetime, Lebwohl said.
Dr. Katy Burris, a dermatologist at North Shore-LIJ Health System in Manhasset, N.Y., found the results impressive.
“Not only was the clearance rate better with brodalumab, but the time it took until clearance was achieved was less when compared to ustekinumab [Stelara],” said Burris.
Burris cautioned, however, that “the long-term safety of this new medication will be determined upon further study and will hopefully be as safe as it is efficacious.”
Side effects from brodalumab included mild to moderate yeast infections, Lebwohl said. These infections were easy to treat and no one stopped the drug because of an infection, he said.
However, two patients committed suicide. “I don’t know of any mechanism why the drug would result in depression or suicide,” he said. “Psoriasis itself increases depression and suicides.”
Assuming the drug is approved by the U.S. Food and Drug Administration, Lebwohl said it will likely be expensive. For comparison purposes, Stelara would cost from $30,000 to $70,000 a year without insurance, according to the National Psoriasis Foundation.
The other study, funded by drug maker Novartis, involved more than 600 patients with psoriatic arthritis.
Participants received either Cosentyx or a placebo drug. About 50 percent of the patients responded to treatment with Cosentyx, compared with a little more than 17 percent of patients receiving placebo, researchers found.
“We have a valuable new asset to treat psoriatic arthritis,” said lead researcher Dr. Philip Mease, a clinical professor of rheumatology at the University of Washington. He said about 30 percent of people with psoriasis will develop psoriatic arthritis.
More information
For more on psoriasis, visit the National Psoriasis Foundation.
Source: HealthDay
Copyright © 2024 HealthDay. All rights reserved.

Leave a Reply